Reactant-Gas-Induced Nanoparticle Dynamics Depend on Size
October 21, 2025
Scientific Achievement
We observe size-dependent morphological dynamics of supported metal nanoparticles (NPs) induced by interaction with reactant gases and find these are correlated with reaction pathway and product selectivity.
Significance and Impact
Supported NPs and NP–support interfaces are key active catalytic sites. It has long been known that their structure and morphology can depend on the identity and concentration of adsorbed reactants and intermediates, and that this dependence can markedly impact catalytic activity. We used state-of-the art environmental-transmission electron microscopy (E-TEM) to follow this dynamic interplay at the individual NP level with atomic resolution and under reaction conditions to uncover transient behaviors relevant to rational catalyst design and optimization.
Research Details
- Developing and optimizing active, selective, and durable catalysts that transform carbon dioxide (CO2) into feedstocks (e.g., carbon monoxide (CO)) and value-added commodities (e.g., carbon nanofibers, methane (CH4), or methanol (CH3OH)), plays a key role in US dominance in processing C1 chemicals (chemicals containing one carbon atom) at scale.
- A rational approach to catalyst development and optimization requires an atomic-level understanding of how the interplay between the catalyst and environment (e.g., reactant concentrations, reaction temperature) affects catalyst performance.
- This study focused on the behavior of cobalt oxide (CoO) nanoparticles (NPs) on ceria (CeO2) nanocubes prepared by incipient wetness impregnation as a catalyst for CO2 conversion to CH4 and CO.
- Catalysts based on cobalt (Co) and CeO2 are attractive because their preparation is straightforward, and both Co and Ce are earth-abundant and inexpensive.
- Catalyst structure, morphology, oxidation state, and interactions with reactants were characterized by a battery of techniques including Environmental transmission electron microscopy (E-TEM), diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), X-ray diffraction (XRD), X-ray absorption fine-structure (XAFS), and X-ray photoelectron spectroscopy (XPS).
- E-TEM was used to characterize the structure (e.g., CoO vs Co3O4) and morphology (size and shape) of individual NP with atomic resolution to examine transient behaviors under reaction conditions during CO2
- Notably, E-TEM revealed:
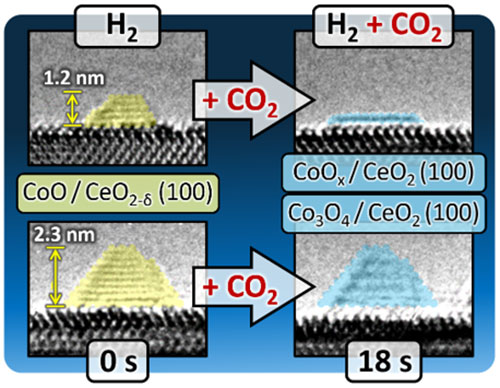
- The CoO NPs exhibit size-dependent morphological dynamics which we attribute to strong interactions with the CeO2(100) surface: When the reactant gas is switched from hydrogen (H2) to a mixture of H2 and CO2 at 250°C, small three-dimensional (3-D) pyramidal CoO NPs (<2.5 nm) are rapidly reduced to CoOx and transformed to two-dimensional (2-D) monatomic layers atop the concurrently oxidized CeO2−δ(100) surface. This maximizes the number of sites available for binding CO2 and reaction intermediates.
- Promptly removing the CO2 from the gas mix reverses the transformation: the CoOx 2-D monatomic layers transition back into 3-D pyramidal CoO NPs.
- Under steady-state conditions, small NPs cycle between the 2-D monatomic structure and a two-layer form.
- At increased CO2 pressures, the 2-D monatomic structures disappear.
- In contrast, no significant morphological changes, apart from oxidation, were observed for larger (>3 nm) CoO NPs.
- The observed morphological and structural changes in the small CoO NPs were correlated with transient increases in the activity for CO2 reduction, adsorbed carbonate (CO3) levels, and CH4
- This study shows that bulk characterization pre- and post-reaction can miss important size-dependent and transient dynamics that impact the performance of this complex but representative system.
- This study focused on cobalt oxide (CoO) NPs on ceria (CeO2) nanocubes and the reduction of carbon dioxide (CO2) to methane (CH4) and carbon monoxide (CO).
- Small (<2.5 nm) CoO NPs transform from 3-D pyramids to 2-D monatomic layers, maximizing the number of available reaction sites when the reactant gas is switched from hydrogen (H2) to a H2 + CO2 mix at 250°C. Subsequent removal of the CO2 results in reversion to the 3-D pyramidal morphology.
- No significant morphological changes are observed for larger CoO NPs.
- Diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) indicates that the morphological changes enhance the catalyst’s selectivity for CH4
Publication Reference
Visualizing Size-Dependent Dynamics of CeO2−δ{100}-Supported CoOx Nanoparticles Under CO2 Hydrogenation Conditions, K. Deng, Y. Wang, P, Perez-Bailac, W. Xu, J. Llorca, A. Martinez-Arias, D.N. Zakharov, J.A. Rodriguez, J. Am. Chem. Soc., 147, online (2025).
DOI: https://doi.org/10.1021/jacs.5c04901
BNL Press Release: https://www.bnl.gov/newsroom/news.php?a=122531
Acknowledgment of Support
This work was supported by the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences (BES), Chemical Sciences, Geosciences, & Biosciences (CSGB) Division, Catalysis Science Program (contract no. DE-SC0012704, FWP CO-040). The E-TEM experiments were conducted at the Electron Microscopy Facility at the Center for Functional Nanomaterials (CFN), a US DOE User Facility operated for the DOE Office of Science by Brookhaven National Laboratory (BNL) under Contract No. DE-SC0012704. X-ray characterization measurements were conducted at the National Synchrotron Light Source II, a US DOE User Facility operated for the DOE Office of Science by BNL under Contract No. DE-SC0012704 and at the Advanced Photon Source, a US DOE User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. J.A.R. acknowledges the support of a DOE Office of Science Distinguished Scientist Fellow Award. J.L. is a Serra Hu´nter Fellow and acknowledges the support of the ICREA Academia program and projects MICINN/FEDER PID2021-124572OB-C31, CEX2023-001300-M, and GC 2021 SGR 01061. P.P.-B. and A.M.-A. acknowledge support under MICINN (Spain) project PID2021-128915NB-I00.
2025-22698 | INT/EXT | Newsroom